Introduction
Overview: The Drug Regulatory Authorities first noticed the presence of the nitrosamine (NSA) impurity, N- Nitrosodimethylamine (NDMA) in products containing valsartan in July 2018. Valsartan is an Angiotensin II Receptor Blocker (ARB) and belongs to a family of analogue compounds commonly referred to as the Sartans. Further, a few other nitrosamines were subsequently detected in other drug substances belonging to the Sartan family, other Active Pharmaceutical Ingredients (API’s), and Finished Pharmaceutical Products (FPP). These included N-nitroso-dimethylamine (NDMA), N-nitroso-N-methyl-4-aminobutyric acid (NMBA), N-nitroso-diethylamine (NDEA), N-ethyl-N-nitroso-isopropylamine (NEIPA), N-nitroso-diisopropylamine (NDIPA) and N- nitroso-di-n-butylamine (NDBA).
Occurrence: Formation of nitrosamines is possible in the presence of secondary, tertiary, or quaternary amines and nitrite salts under acidic reaction conditions. Under these conditions, nitrite salts may form nitrous acid, which can react with an amine to form a nitrosamine. Apart from these there are other routes such as; vendor-sourced starting materials and raw materials; recovered solvents, catalysts and reagents; cross contamination from common manufacturing facility; quenching process using nitrous acid; and packing/storage; which may result in nitrosamines formation or contamination.
Toxicity/ Regulation/ Methods: NDMA and NDEA belong to the so-called “cohort of concern”, which is a group of highly potent mutagenic carcinogens that have been classified as probable human carcinogens. Hence, United States Food and Drug Administration (USFDA) recommends the following acceptable intake (AI) limits for NDMA, NMBA, NDEA, NEIPA, NMPA and NDIPA (Table 1). These limits are applicable only if a drug product contains a single nitrosamine, and lowest of which is 0.03 ppm for drug substances (DS) with Maximum daily dose (MDD) of 880 mg/day. If more than one nitrosamine impurity is identified in the same DS the limit for total nitrosamines listed in Table 1 is still not more than 26.5 ng/ day or 0.03 ppm. Hence, it is imperative to detect above mentioned NSA’s with Limit of Quantitation (LOQ) as low as possible to be sure that not just single nitrosamine impurity is below 0.03 ppm, but also total nitrosamine impurities are below 0.03 ppm.
| Nitrosamine | AI Limit (ng/day) | Limit in ppm for MMD 880 mg/day |
| NDMA | 96.0 | 0.109 |
| NDBA | 96.0 | 0.109 |
| NDEA | 26.5 | 0.030 |
| NEIPA | 26.5 | 0.030 |
| NMPA | 26.5 | 0.030 |
| NDIPA | 26.5 | 0.030 |
Table 1 . Acceptable Intake (AI) Limits for Nitrosamines
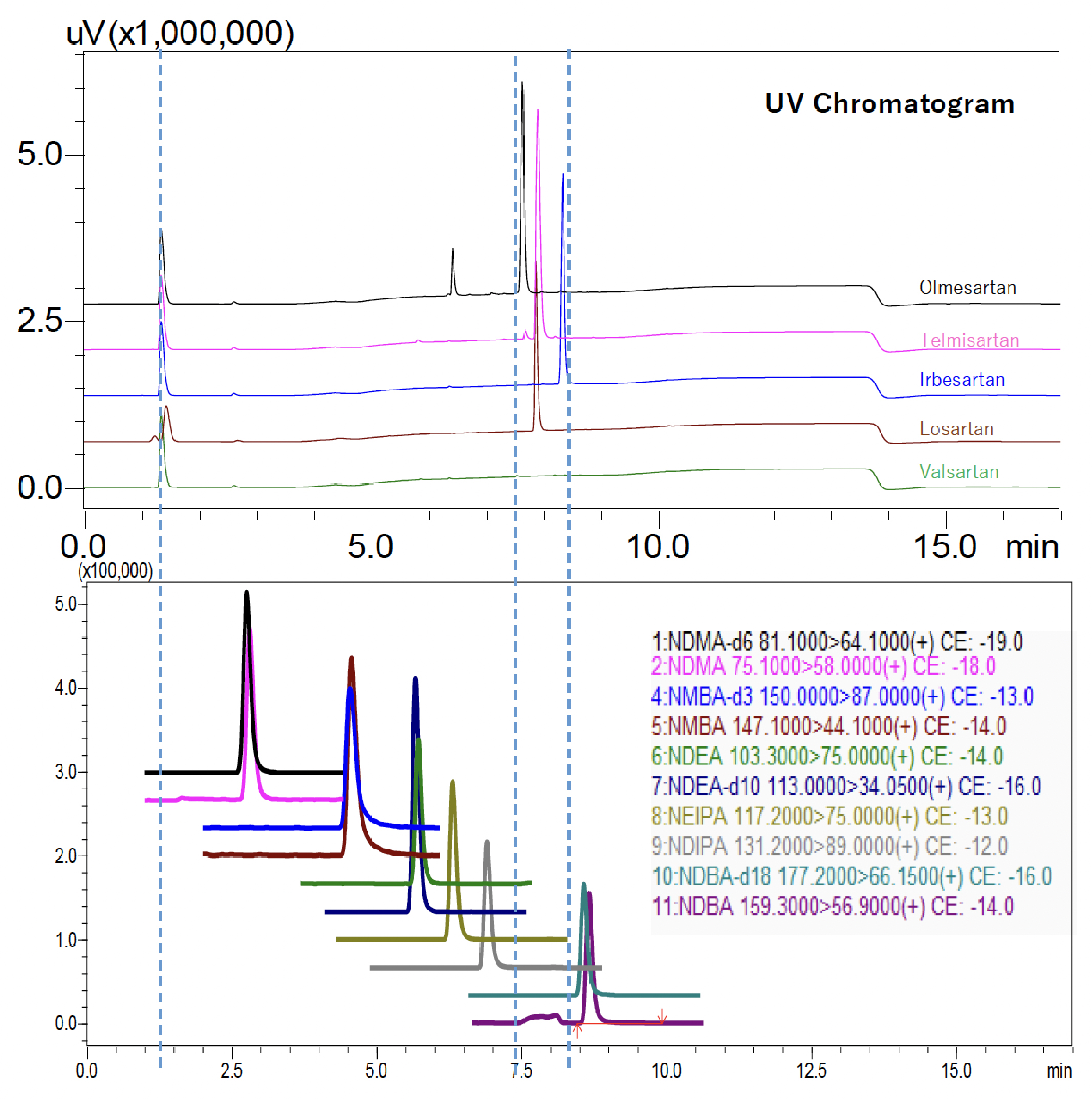
The United States Pharmacopeia (USP) published the proposed General Chapter <1469> which has been used for the quantification of nitrosamines in 5 different Sartans namely Olmesartan, Telmisartan, Irbesartan, Losartan and Valsartan. In this application note “procedure 3” has been performed on LCMS-8045 system by using Raptor ARC-18 (150mm X 3.0mm x 2.7 µm).
Experiment
Six nitrosamines namely NDMA, NMBA, NDEA, NEIPA, NDIPA and NDBA were analyzed using Ultra High Performance Liquid Chromatography (UHPLC) NexeraTM XS coupled with LCMSTM-8045, a triple quadrupole mass spectrometer from Shimadzu Corporation, Japan (Fig. 1).
LCMS-8045, sets a new benchmark in triple quadrupole technology with an unsurpassed sensitivity (UFsensitivity), ultra fast scanning speed of 30,000 u/sec (UFscanning) and polarity switching speed of 5 msec (UFswitching). This system ensures highest quality of data, with very high degree of reliability.
All six nitrosamines are mid polar compounds. They were easily ionized by Atmospheric Pressure Chemical Ionization (APCI) interface.

Method
The MRM transitions of 6 nitrosamines and 4 internal standards are given in Table 2 and analytical conditions in Table 3.
| MRM Transitions |
|||
| Nitrosamine Impurity | Acquisition Mode | Polarity | MRM (Quantifier, Qualifier) |
| NDMA | MRM | Positive | 75>43, 75>44 |
| NDMA-d6 | MRM | Positive | 81>46, 81>64 |
| NMBA | MRM | Positive | 147>44, 147>117 |
| NMBA-d3 | MRM | Positive | 150>47, 150>120 |
| NDEA | MRM | Positive | 103>75, 103>47 |
| NDEA-d10 | MRM | Positive |
113>34, 113>49
|
| NEIPA | MRM | Positive | 117>75, 117>45 |
| NDIPA | MRM | Positive | 131>89, 131>47 |
| NDBA | MRM | Positive | 159>41, 159>29 |
| NDBA-d18 | MRM | Positive | 177>66, 177>46 |
Table 2 MRM Transitions of Nitrosamines
Table 3 Analytical Conditions
HPLC System: Nexera XS
Column: Raptor ARC-18 (150 mm x 3.0 mm x 2.7 µm)
Column Oven: 60 °C
Mobile Phases: A-0.1 % Formic Acid in Water, B-0.1 % Formic Acid in Methanol
Flow Rate: 0.5 mL/min
Gradient Program: 0-1.5 min → 3 (%); 1.5-4 min → 3-50 (%); 4-7 min → 50-75 (%); 7-8.1 min → 75-85 (%); 8.1-9.2 min → 85-95 (%); 9.2-12 min → 95 (%); 12-12.1 min → 95-3 (%); 17 min → STOP
Injection Volume: 20µL
LCMS System: LCMS-8045, APCI
Temperature: Interface: 270 °C, Desolvation Line: 220 °C, Heater Block: 220 °C
Gas Flow: Nebulizing Gas: 3L/min, Drying Gas: 3 L/min
A representative LCMS chromatogram for nitrosamines with the UV chromatogram of sartans is given in Fig 2. A divert valve was employed to direct all API peaks to waste and to protect LCMS from the contaminations.
Linearity of The Nitrosamines
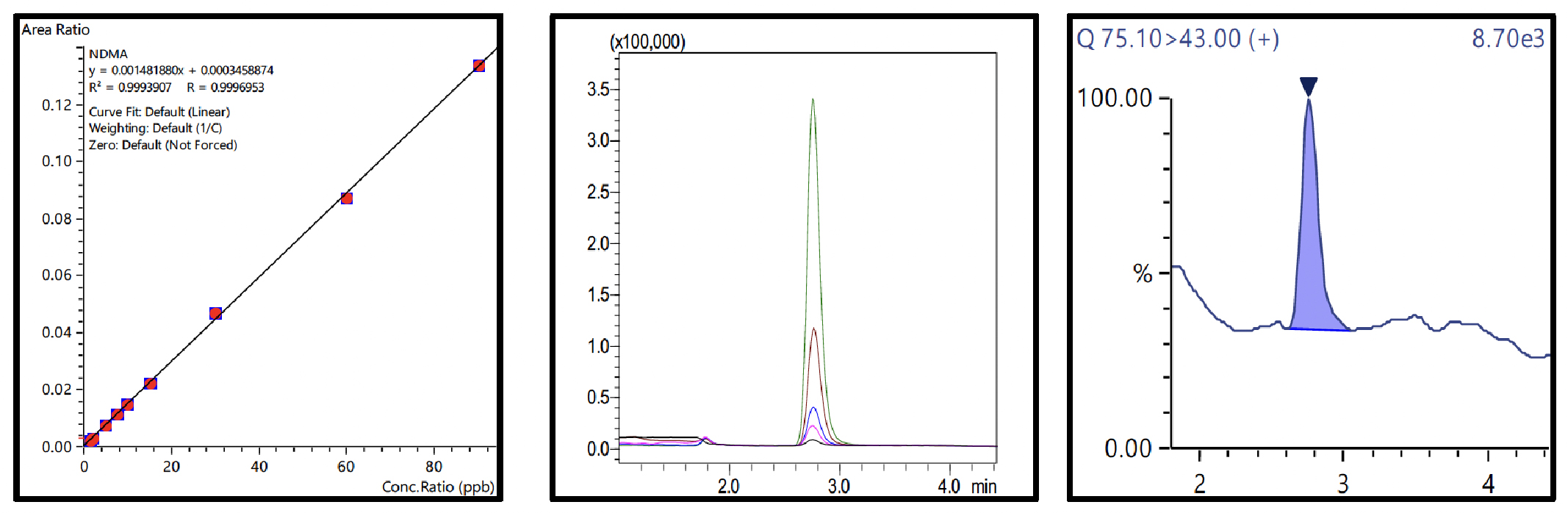
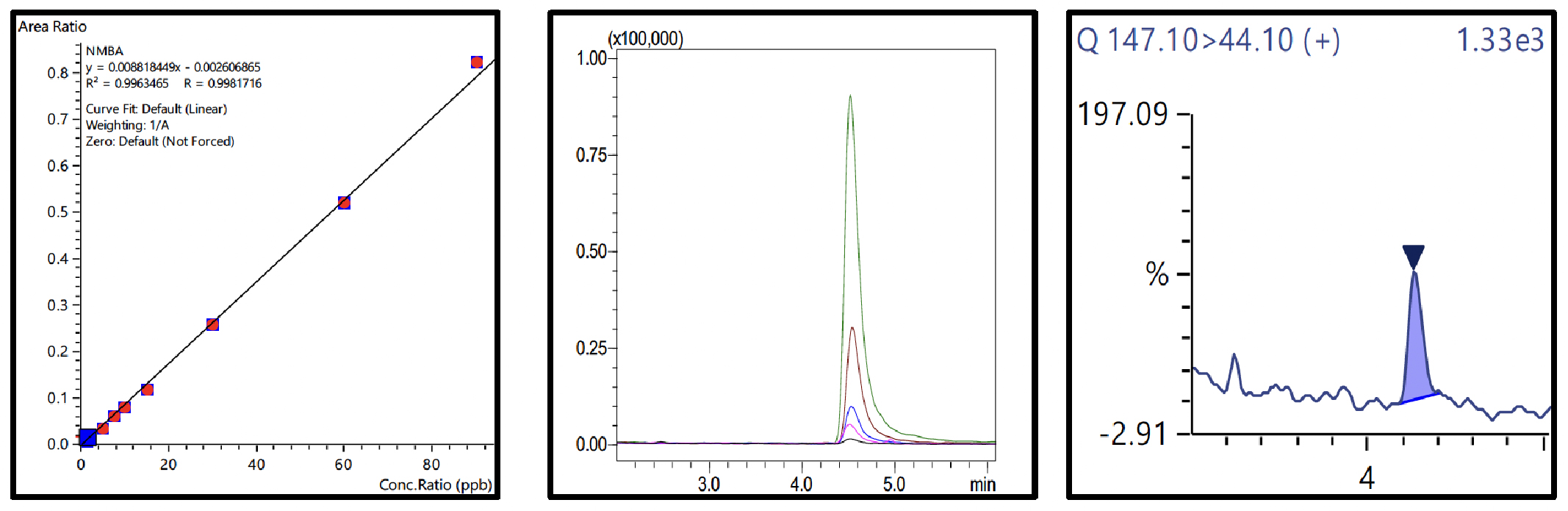
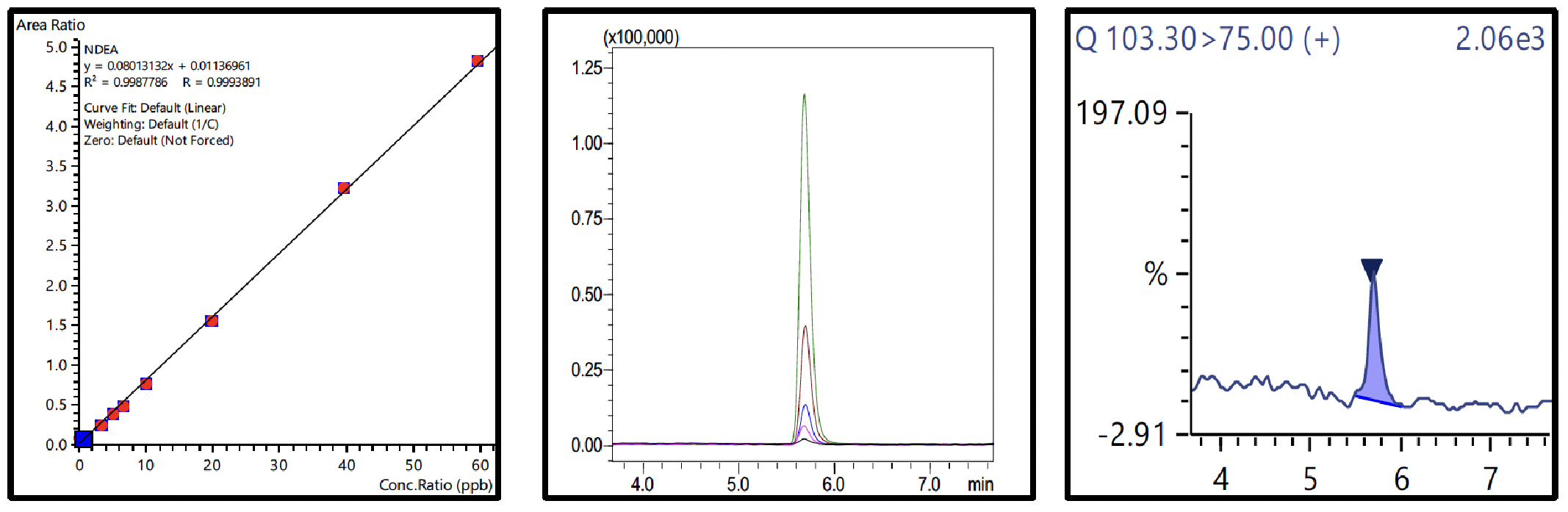
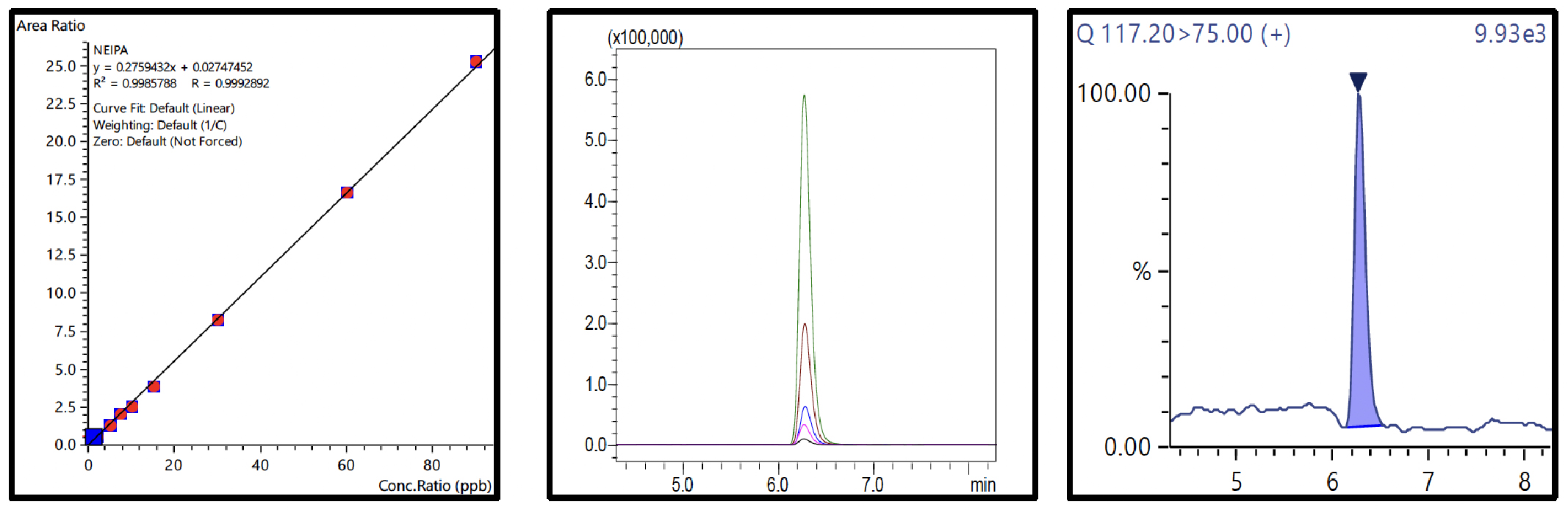
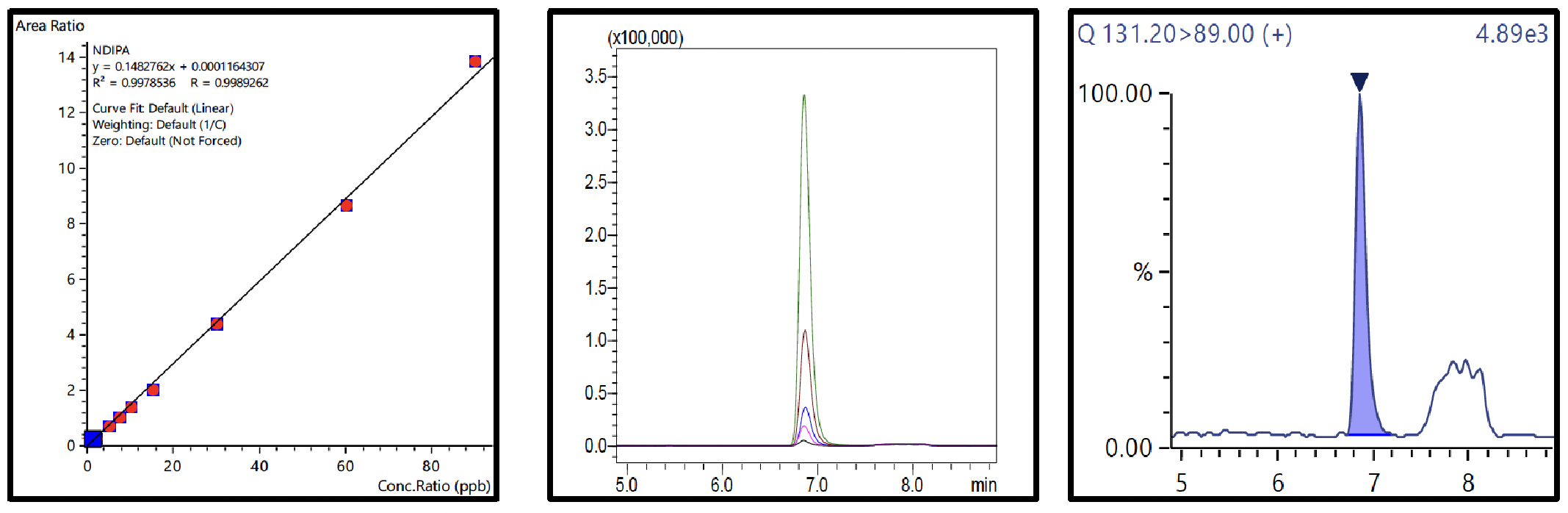
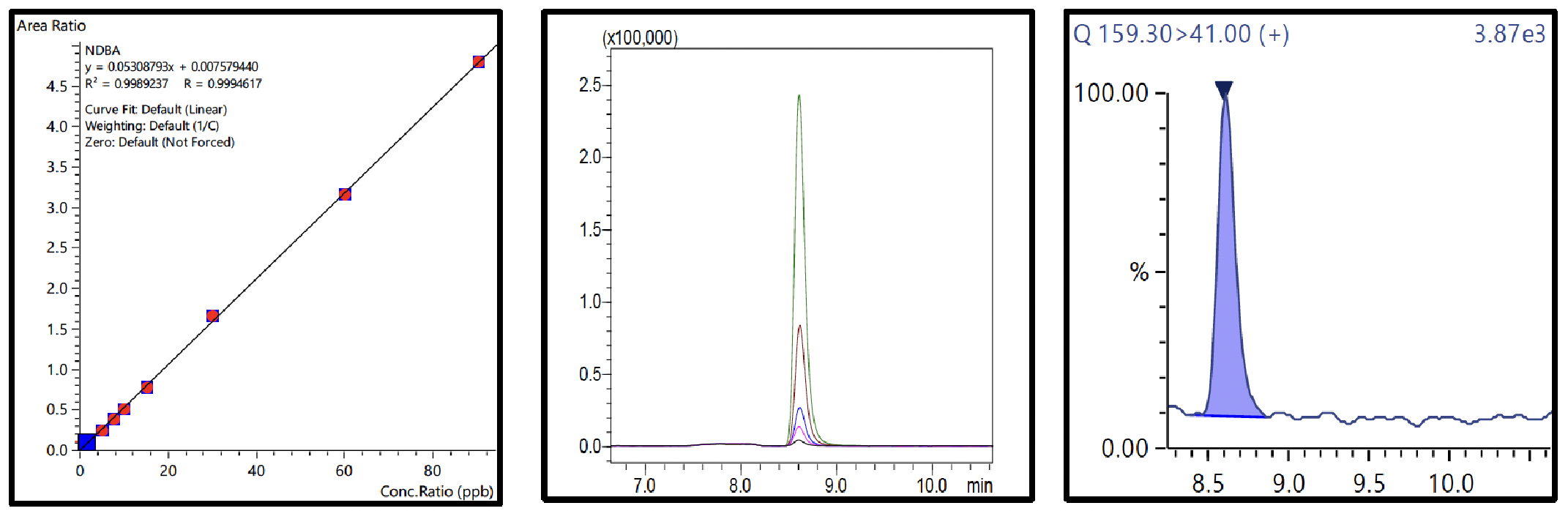
Nine-point calibration curves for all 6 NSAs with 4 ISTDs were prepared in 1% formic acid in water and analyzed using the conditions described in Table 3. The Fig.3 to 8 depicts the calibration curves, overlay of linearity standards & LOQ solution chromatograms for NDMA, NMBA, NDEA, NEIPA, NDIPA, & NDBA, respectively.
| Comp. |
CC Range (ppb) |
R2 |
LOQ |
||
| Conc. (ppb) | %RSD (n=6) | S/N | |||
| NDMA | 1.33 to 90 |
0.999 | 1.33 |
2.68 | 25.34 |
| NMBA | 0.998 | 15.66 | 15.08 | ||
| NEIPA | 0.999 | 10.3 | 40.19 | ||
| NDIPA | 0.998 | 1.85 | 90.03 | ||
| NDBA | 0.999 | 3.66 | 89.85 | ||
| NDEA | 0.66 to 59.4 | 0.999 | 0.66 | 5.90 | 29.84 |
Table 4 Summary of Calibration Curves
The range for calibration curves, LOQ established from S/N and % RSD at LOQ are shown in Table 4.
Sample Analysis
Weigh 80 mg of sample drug substance in 2 mL centrifuge tube , then add 1188 µL µLof diluent and 12 µL of internal standard. Vortex at 2500 rpm for 20 min, except for losartan (vortex losartan NMT 5 min). Then centrifuge at 10,000 rpm for 10 minutes. Filter the supernatant through 0.22-µm hydrophilic PTFE syringe filter and inject the sample for LC- MS analysis.
The overall concentration was 66.6 mg/mL. The results of the sample spiked study (recovery study) for the five sartans at LOQ level are given in Table 5.
| % Recovery at 0.03 ppm for 5 NSAs and 0.014 ppm for NDEA | |||||
| Name | Olmesartan | Telmisartan | Irbesartan | Losartan | Valsartan |
| NDMA | 90.7 | 86.9 | 114.2 | 95.7 | 92.1 |
| NMBA | 117.7 | 114.5 | 108 | 112.6 | 118.7 |
| NEIPA | 94.5 | 93.7 | 94.4 | 93.7 | 93.7 |
| NDIPA | 100.7 | 105.5 | 98.0 | 81.4 | 86.1 |
| NDBA | 104.5 | 110.5 | 115.2 | 108.7 | 111.8 |
| NDEA | 71.8 | 73.2 | 99.6 | 77.1 | 86.8 |
Table 5 The sample spiked study for 5 sartans at LOQ level
Note: Criteria for % Recovery as per USP <1469> is 70 to 130%.
Conclusion
- A single LC-MS/MS quantification method for six nitrosamines in 5 sartans as per proposed USP general chapter <1469> (procedure-3) has been successfully carried out with the Shimadzu LCMS-8045 system.
- Nine levels of linearity were performed for all the six nitrosamines by internal standard method.
- Correlation coefficient was greater than 0.99 for all the six nitrosamines.
- The repeatability (n=6) at LOQ level was found to be less than 25% RSD.
- Recovery analysis was performed at LOQ level in all five sartans and it matched to the acceptance criteria between 70 to 130 %.