Introduction
Mutual interactions between the pharmaceutical and packaging are a problem with pharmaceutical packaging materials that requires particular care. Extractables are compounds generated from storage conditions that are more severe than normal, whereas leachables are compounds that can be transferred from the packaging to the pharmaceutical under normal storage conditions. They are classified as indicated in Table 1. Before pharmaceuticals can be sold, extractables and leachables must be comprehensively verified to determine packaging material risks.
| Extractables | Leachables | |
| Description | All compounds that potentially could be extracted from packaging | Compounds that leach out under normal usage conditions |
| Extraction Conditions | More severe conditions than for normal use or storage. High-temperature extraction or solvent extraction is used. | Under normal conditions for use or storage. |
| Measured Item | Packaging material | Pharmaceutical |
Table 1 Summary of Extractables and Leachables
Background of Extractables and Leachables in Society
Despite the variety of regulations governing extractables and leachables, still no definitive method of analysis has been specified. Most regulations indicate that complete measurement of extractables and leachables is impossible.
For example, USP 1663 says “It is not possible for a general discussion of extractables to anticipate and cover all situations where an extractables assessment might be required.”
However, in July 2020, the ICH launched a new working group for evaluating and managing extractables and leachables (ICH- Q3E). The increase in biopharmaceuticals is the main reason the working group was started. The FDA summarized the potential interactions between pharmaceuticals and packaging materials in “Guidance for Industry: Container Closure Systems for Packaging Human Drugs and Biologics.” That guidance indicates an increased danger to humans from parenterally administered drugs or parenterally administrable drug suspensions (Table 2). Given that biopharmaceuticals comprise macromolecules that make oral administration difficult, most are administered parenterally. As of 2020, biopharmaceuticals account for about 30 % of the overall pharmaceuticals market. Consequently, the evaluation of extractables and leachables can no longer be ignored.
Furthermore, because of the specificity of biopharmaceuticals they are often only effective for rare diseases, so the cost- effectiveness of scaling up is low. Therefore, rather than using a stainless steel culture tank or other large-scale manufacturing equipment, there is a growing trend toward successively producing a variety of small batches. Due to the plastic single- use equipment used for production, the risk of leachables being generated between the equipment and pharmaceuticals must also be taken into consideration.
| Degree of Concern Associated with the Route of Administration | Likelihood of Packaging Component-Dosage Form Interaction | ||
| High | Medium | Low | |
| Highest | Inhalation Aerosols and Solutions; Injections and Injectable Suspensions | Sterile Powders and Powders for Injection; Inhalation Powders | |
| High | Ophthalmic Solutions and Suspensions; Transdermal Ointments and Patches; Nasal Aerosols and Sprays | ||
| Low | Topical Solutions and Suspensions; Topical and Lingual Aerosols; Oral Solutions and Suspensions | Topical Powders; Oral powders | Oral Tablets and Oral (Hard and Soft Gelatin) Capsules |
Table 2 Examples of Packaging Concerns for Common Classes of Drug Products
Source: Guidance for Industry Container Closure Systems for Packaging Human Drugs and Biologics
Samples and Analytical Conditions
For this article, plastic bags for liquid formulations were used as samples. The bags were made of polyvinyl chloride (PVC). GC- MS was used to identify volatile and semi-volatile extractable compounds in the samples. The extractables were pretreated by extraction using various solvents and by high-temperature extraction using a headspace sampler without any solvent.
For solvent extraction, dichloromethane (DCM) and hexane, commonly used to extract plastic components, and ethanol, commonly used in pharmaceutical manufacturing, were used as the extraction solvents. About 400 mg of the samples cut into 1 cm squares were weighed into vials containing 5 mL of respective solvents, and then the vials were sealed. After ultrasonically treating the vials for 5 hours, the vials were left at room temperature for 3 days for extraction. Then the organic solvent supernatant was analyzed by GC-MS. The analytical conditions are indicated in Table 3.
For high-temperature extraction, about 200 mg of the samples cut into 1 cm squares were weighed into headspace vials purged with nitrogen gas and then sealed. Because the vials are heated to temperatures over 200 °C for high-temperature extraction, they were sealed with heat-resistant headspace vial septa that minimize bleeding at high temperatures (Shimadzu, P/N: 225-40950-91). The analytical conditions are indicated in Table 4.
For high-temperature extraction, n-alkane was also analyzed to enable calculation of retention index values for each peak by qualitative analysis using the Polymer Additives Library. 5 μL of an n-alkane solution (1000 μg/mL hexane solvent) was sealed in a headspace vial purged with nitrogen gas and similarly analyzed.
Table 3 Analytical Conditions (Solvent Extraction: Liquid Injection-GC/MS
Instruments
Autosampler: Shim-pack Bio IEX SP-NP (4.6 mm x 100 mm; 3 μm)
GC-MS: GCMS-QP2020 NX
Column: SH-I-5MS (30 m × 0.25 mm I.D., df = 0.25 μm)
GC Conditions
Injection Unit Temp.: 250 °C
Injection Mode: Splitless
Carrier Gas: Helium
Control Mode: Constant Linear Velocity (36.1 cm/s)
Oven Program: 40 °C (3 min) → 10 °C/min → 330 °C (15 min)
Injection Volume: 1 μL
MS Conditions
Interface Temp.: 300 °C
Ion Source Temp.: 230 °C
Ionization Method: EI
Measurement Mode: Scan (m/z 29 to 800)
Event Time: 0.3 sec
Table 4 Analytical Conditions (High-Temperature Extraction: Headspace-GC/MS)
Instruments
Autosampler: HS-20
GC-MS: GCMS-QP2020 NX
Column: SH-I-5MS (30 m × 0.25 mm I.D., df = 0.25 μm)
Headspace (HS) Conditions
Mode: Loop
Over Temp.: 250°C
Sample Line Temp.: 250°C
Transfer Line Temp.: 250°C
Vial Pressure: 150kPa
Vial Heating Time: 10 min (1 min for n-alkane)
Vial Pressurization Time: 1min
GC Conditions
Injection Mode: Split (split ratio: 10)
Carrier Gas: Helium
Control Mode: Constant Linear Velocity (36.1 cm/s)
Oven Program: 40 °C (3 min) → 10 °C/min → 330 °C (15 min)
MS Conditions
Interface Temp.: 300 °C
Ion Source Temp.: 230 °C
Ionization Method: EI
Measurement Mode: Scan (m/z 29 to 800)
Event Time: 0.3 sec
Solvent Extraction Analytical Results
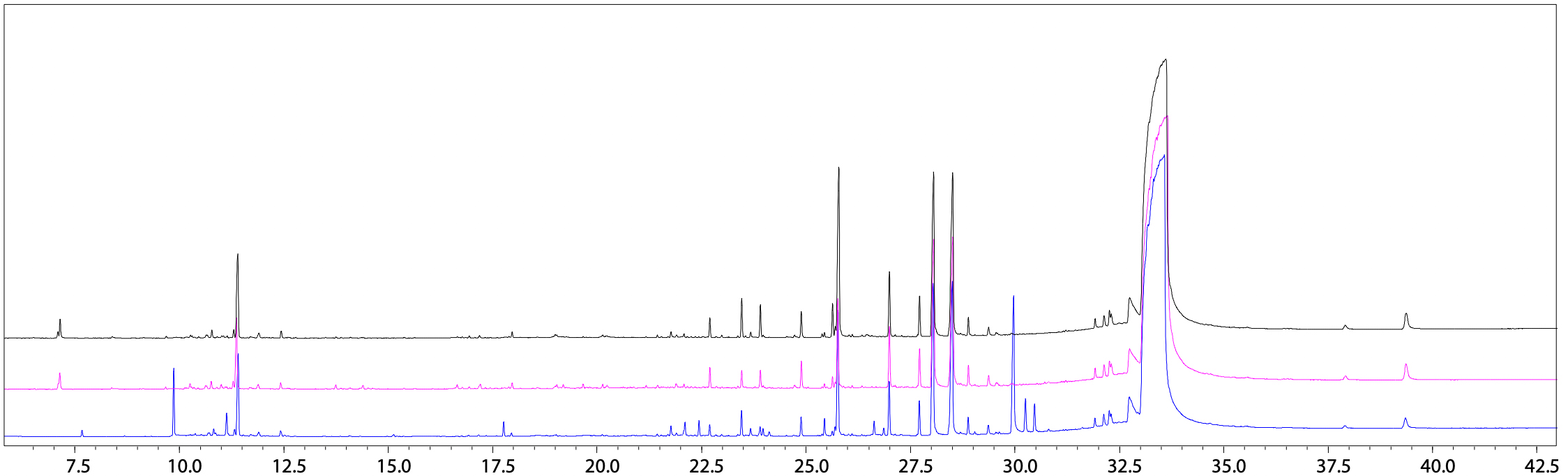
Total ion current chromatograms (TICC) obtained from analyzing hexane, DCM, and ethanol extract solutions are shown in Fig. 1. Compounds were identified by using the NIST Library (2020 version) and the Polymer Additives Library.
Compounds detected using respective extraction solvents are indicated in Table 5. They include plasticizers, such as tris(2- ethylhexyl)trimellitate (TOTM), di(2-ethylhexyl)phthalate (DEHP), and di(2-ethylhexyl)adipate (DEHA), and lubricants, such as palmitic acid and ethyl palmilate. However, there was some difference in the components detected with each extraction solvent. That difference in detected components is presumably due to differences in the polarity of respective extraction solvents. In general, hexane and other solvents with low polarity are more effective in extracting compounds with low polarity, whereas ethanol and other solvents with high polarity are more effective in extracting compounds with high polarity. In this example, most compounds were detected by extraction using ethanol.
| Compound | Hexane | DCM | Ethanol | Remarks |
| R.T. (min) | R.T. (min) | R.T. (min) | ||
| 2-Ethylhexanol | - | - | 9.86 | |
| Isophorone | 11.37 | 11.40 | 11.40 | Solvent |
| Palmitic acid | - | 21.77 | 21.77 | Lubricant |
| Ethyl palmitate | - | - | 22.10 | Lubricant |
| 2-Ethylhexyl methyl isophthalate | 23.46 | 23.46 | 23.46 | |
| Stearic acid | - | 23.67 | 23.67 | Lubricant |
| Butyl palmitate | - | 23.91 | 23.90 | Lubricant |
| Ethyl stearate | - | 23.90 | 23.97 | Lubricant |
| Terephthalic acid, ethyl 2-ethylhexyl ester | - | - | 24.12 | |
| Methyl 9,10-epoxystearate | 24.89 | 24.89 | 24.88 | |
| Butyl stearate | - | 25.63 | - | Lubricant |
| Di(2-ethylhexyl) adipate | 25.75 | 25.78 | 25.78 | Plasticizer (DEHA) |
| Ethyl stearate, 9,12-diepoxy | - | - | 26.63 | |
| Di(2-ethylhexyl) phthalate | 27.00 | 26.99 | 26.99 | Plasticizer (DEHP) |
| Bis(2-ethylhexyl) isophthalate | 28.03 | 28.05 | 28.04 | Plasticizer |
| Bis(2-ethylhexyl) terephthalate | 28.48 | 28.51 | 28.50 | Plasticizer |
| Epoxidized 2-ethylhexyl oleate | 29.37 | - | 29.36 | |
| Tris(2-ethylhexyl) Trimellitate | 33.67 | 33.65 | 33.59 | Plasticizer (TOTM) |
Table 5 Compounds Detected by Solvent Extraction
Analytical Results Using High-Temperature Extraction
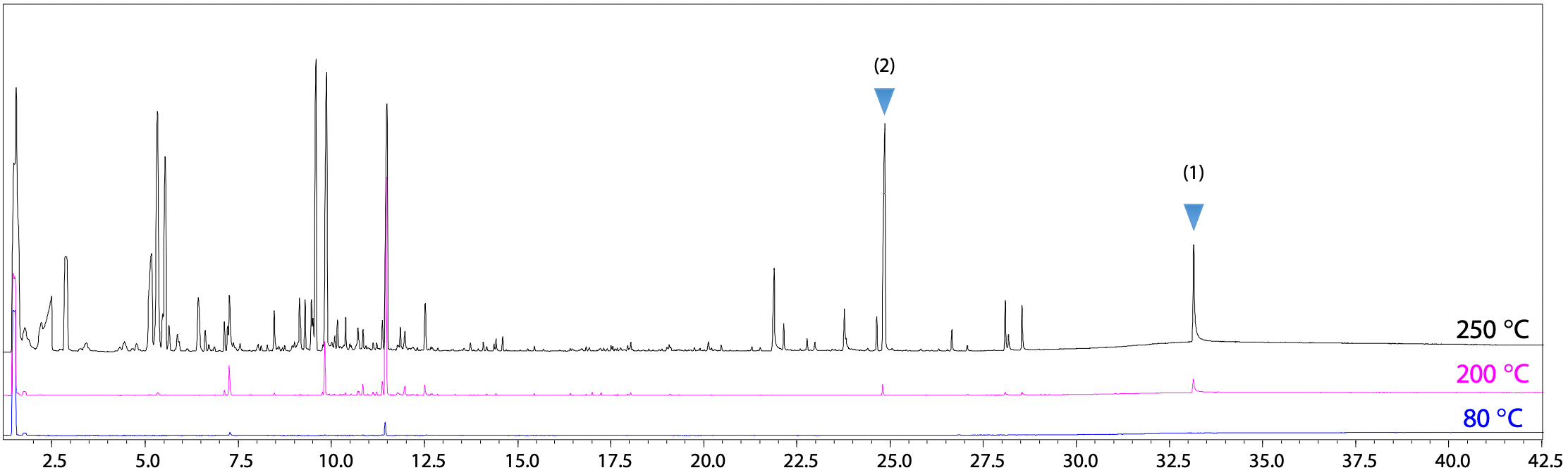
Headspace-GC-MS was used for high-temperature extraction. For high-temperature extraction using a headspace sampler, samples can be analyzed after simply placing them into vials and loading the vials into the sampler, which offers the advantage of extremely simple pretreatment. First, to determine the optimal extraction temperature, the vial temperatures were varied by heating to 80, 100, 150, 200, and 250 °C to compare the results. TICCs for 80, 200, and 250 °C vial temperatures are shown in Fig. 2. In this example, a vial temperature of 250 °C was determined to provide the highest compound extraction efficiency, because it resulted in detecting the most peaks.
High-temperature extraction using headspace-GC-MS offers the advantage of simple pretreatment, but thermal decomposition of extracted components can result in more complex chromatograms that are more difficult to analyze. Therefore, the Polymer Additives Library was used in addition to the NIST Library as mass spectral libraries for compound identification. The Polymer Additives Library includes mass spectra for a wide range of additives used in polymer materials and corresponding additive decomposition products. Retention index information is registered for each compound to enable more accurate compound identification by filtering results based on retention indices.
It also includes additive classification information, so that additives can be analyzed more easily even without extensive knowledge of additives.
As an example of using the Polymer Additives Library for qualitative analysis, Fig. 3 shows compounds identified for peaks (1) and (2) in Fig. 2. Peak (1) was identified TOTM based on mass spectral similarity and retention indices for polymer additives. In contrast, a search for peak (2) generated no hits from the general purpose NIST Library, but by using the Polymer Additives Library it was predicted to be a decomposition product of TOTM, or in other words, a decomposition product of peak (1).
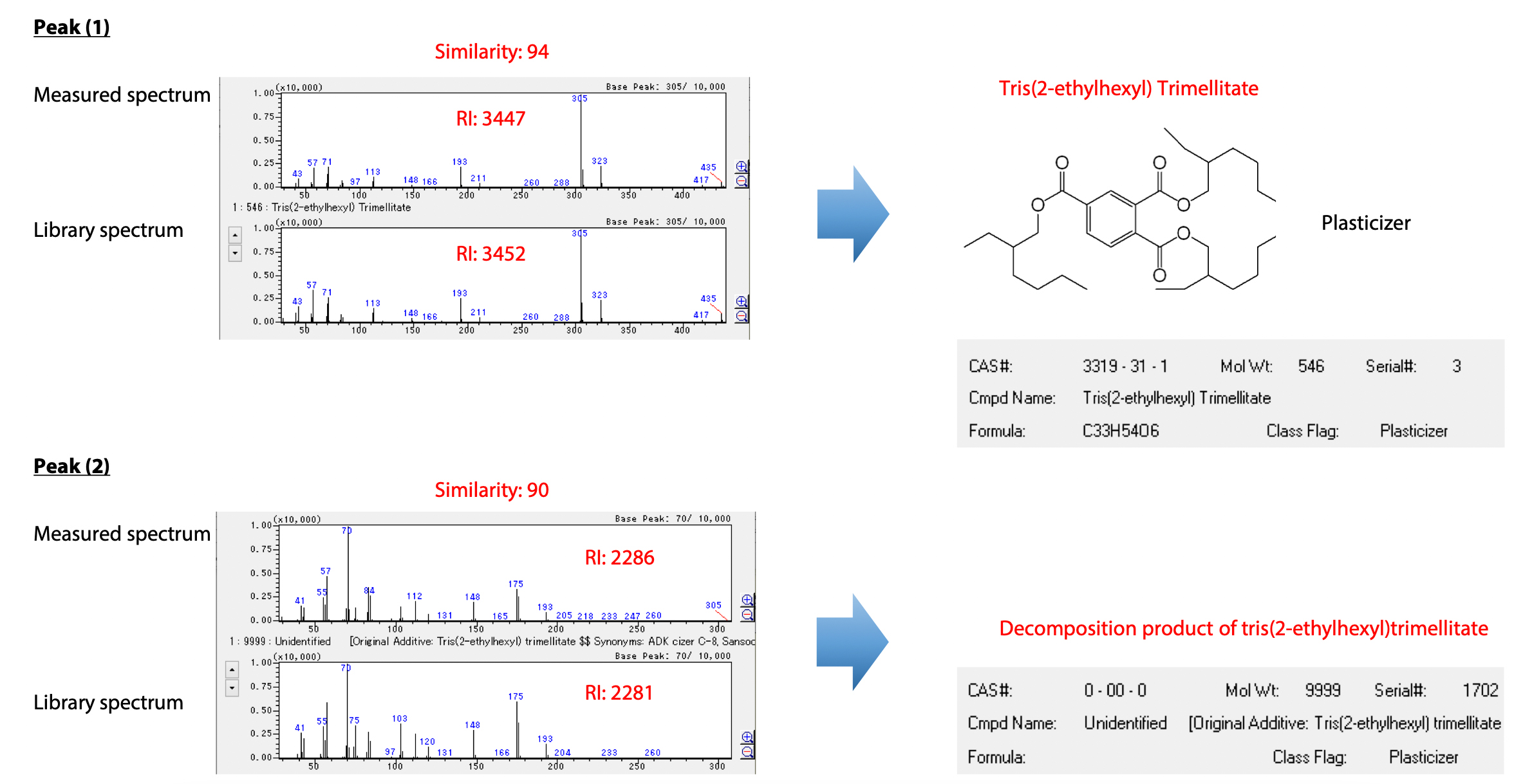
Thus, the Polymer Additives Library can be used to easily analyze even decomposition products of extractables detected by high-temperature extraction. The compounds detected by high-temperature extraction are indicated in Table 6.
Conclusion
Extractables from pharmaceutical packaging materials were identified by both solvent and high-temperature extraction methods. By using a variety of solvents, solvent extraction can be used for compounds with a wide range of properties. By using a headspace sampler with high-temperature extraction, samples can be pretreated more simply and quickly than for solvent extraction. For data analysis, extractables can be identified accurately and easily by using the Polymer Additives Library. The above examples confirm that the Polymer Additives Library can be used in combination with headspace-GC-MS to easily screen extractables from pharmaceutical packaging materials.
| Compound | R.T. (min) | Remarks |
| Benzene | 2.87 | Solvent |
| 2-Ethyl-1-hexene | 5.52 | Decomposition products of TOTM |
| 2-Chloro-octane | 9.27 | Decomposition products of PVC |
| 3-(Chloromethyl)heptane | 9.57 | Decomposition products of PVC |
| 2-Ethylhexanol | 9.85 | Decomposition products of TOTM |
| Isophorone | 11.47 | Solvent |
| Palmitic acid | 21.85 | Lubricant |
| Stearic acid | 23.73 | Lubricant |
| Butyl palmitate | 23.95 | Lubricant |
| Unidentified | 24.80 | Decomposition products of TOTM |
| Di(2-ethylhexyl) adipate | 25.79 | Plasticizer (DEHA) |
| Di(2-ethylhexyl) phthalate | 27.03 | Plasticizer (DEHP) |
| Bis(2-ethylhexyl) isophthalate | 28.05 | Plasticizer |
| 2-Ethylhexyl stearate | 28.14 | |
| Bis(2-ethylhexyl) terephthalate | 28.50 | Plasticizer |
| Tris(2-ethylhexyl) Trimellitate | 33.10 | Plasticizer (TOTM) |
Table 6 Examples of Compounds Detected by High-Temperature Extraction