Introduction
Pharmaceutical water used in drug manufacturing processes must have a high level of safety and minimal impurities. Total organic carbon (TOC) is used in control of organic impurities in pharmaceutical water, and control standards corresponding to the application are provided in the pharmacopoeias used in various countries. For example, the Japanese Pharmacopoeia (JP) sets a limit of “not more than 0.500mg/L” for TOC in purified water and water for injection (bulk). Quick and easy control of organic impurities is possible with Shimadzu TOC analyzers, even in cases where measurement with this high sensitivity/high accuracy is required.
Application News No. O81 introduced online measurement of ultrapure water with the TOC-1000e. This article introduces an example of measurement of pharmaceutical water with the combustion catalytic oxidation type TOC-L and the wet chemical oxidation type TOC-V, which enable batch measurement in the laboratory.
Measurement Principle: Oxidation Methods
TOC analyzers generally decompose organic substances in a sample by oxidation and measure the generated carbon dioxide. Methods of oxidative decomposition are broadly divided into the combustion catalytic oxidation method and the wet chemical oxidation method. Table 1 shows their respective features and main applications.
Combustion catalytic oxidation has high oxidizability and enables oxidative decomposition regardless of the type and state of existence of organic substances. For this reason, it is the optimum method for measurements of environmental water and waste water, but since this method also realizes high sensitivity, which a limit of detection of 4 μg/L, it amply satisfies the requirements for measurement of pure water. The wet chemical oxidation method provides higher sensitivity measurement performance and is the optimum method for measurements of ultrapure water.

| Wet chemical oxidation | Combustion catalytic oxidation | |
| Features | High sensitivity
|
High oxidizability
|
| Main applications | Ultrapure water, pure water (pharmaceutical water), tap water | Pure water (pharmaceutical water), tap water, environmental water, waste water |
Table 1 Oxidation Methods
Analysis Method
Pure water samples were measured under the conditions in Tables 2 and 3 using a combustion catalytic oxidation type TOC-L and a wet chemical oxidation type TOC-V.
| Instrument : | Total organic carbon analyzer TOC-LCPH |
| Oxidation method : | 680 ˚C combustion catalytic oxidation |
| Catalyst : | High sensitivity catalyst |
| Measurement items : | NPOC (non-purgeable organic carbon; TOC measurement by acidification and sparging) |
| Calibration curve : | 2-point calibration curve by 0-500 μgC/L KHP (aq) |
| Injection volume : | 2000 μL |
Table 2 Measurement Conditions of Combustion Catalytic Oxidation Type TOC-L
| Instrument : | Total organic carbon analyzer TOC-VWP |
| Oxidation method: | Oxidation by oxidant, UV irradiation, and heating |
| Oxidant : | Sodium peroxodisulfate |
| Measurement items : | NPOC (TOC measurement by acidification and sparging) |
| Calibration curve : | 2-point calibration curve by 0-500 μgC/L KHP (aq) |
| Injection volume : | 3000 μL |
Table 3 Measurement Conditions of Wet Chemical Oxidation Type TOC-V
Calibration Curve
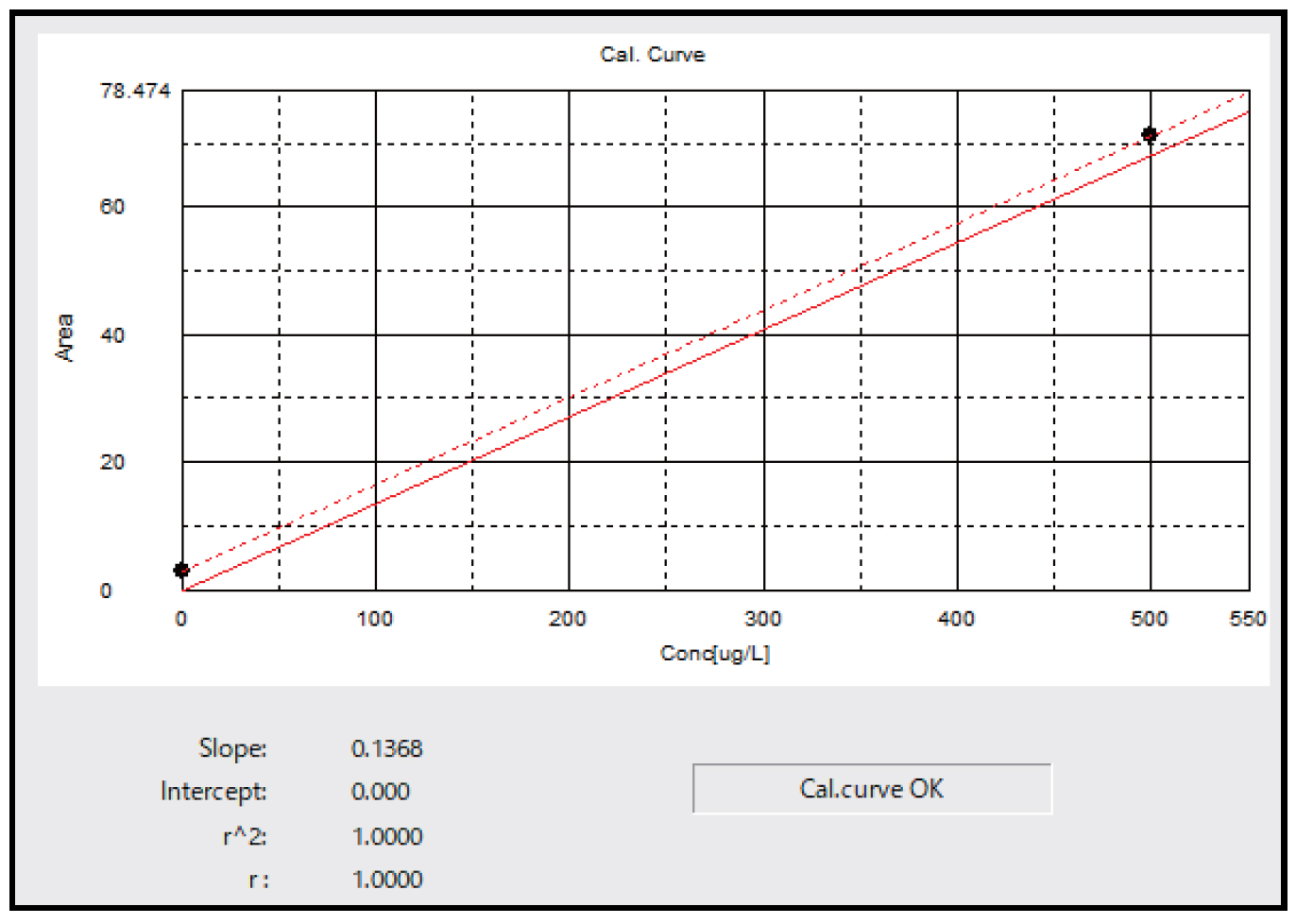
Fig. 2 shows the 2-point calibration curve prepared using 0 and 500 μgC/L (=ppb) potassium hydrogen phthalate (KHP). In order to eliminate the effect of carbon impurities in the ultrapure water used to prepare the standard solutions, the calibration curve was shifted in parallel so as to pass through the coordinate origin.
Measurement Results
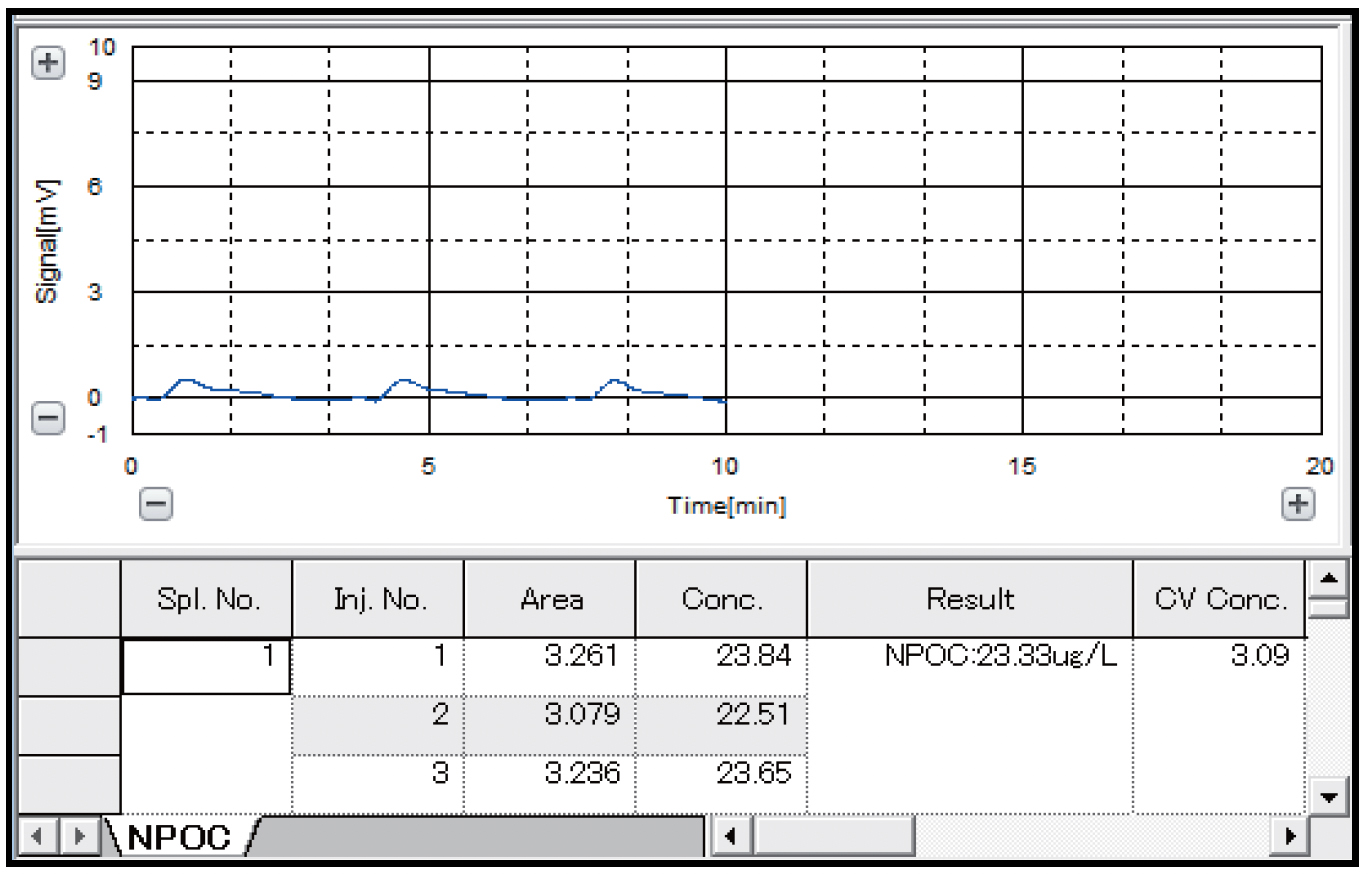
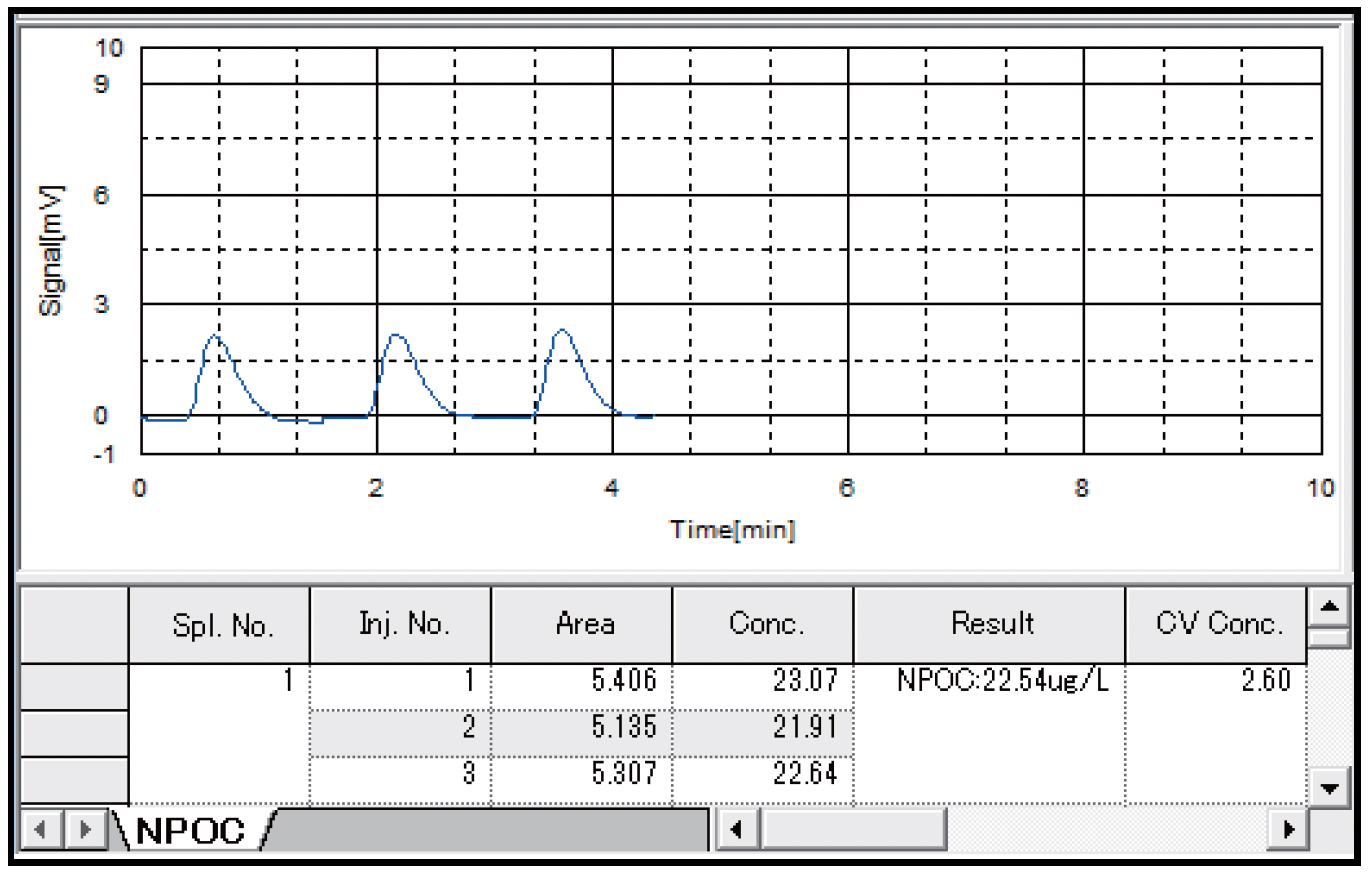
Table 4 shows the results of measurements of pure water with the combustion catalytic oxidation type TOC-L and the wet chemical oxidation type TOC-V, and Figs. 3 and 4 show the measurement charts. Similar TOC concentration values of 23.33 μgC/L and 22.54 μgC/L were obtained by the combustion catalytic oxidation method and the wet chemical oxidation method, respectively. In both cases, measurement with good repeatability was possible, as the coefficient of variation (CV) was approximately 3% or less.
| Oxidation method | Combustion catalytic oxidation type TOC-L | Wet chemical oxidation type TOC-V |
| Result (μgC/L) | 23.33 | 22.54 |
| Coefficient of variation (%) | 3.09 | 2.60 |
Table 4 Measurement Results
Conclusion
In this article, TOC measurements of pure water were carried out with a combustion catalytic oxidation type TOC-L and a wet chemical oxidation type TOC-V. As the measurement results, similar TOC concentration values were obtained with both instruments, and the repeatability of the measurements was also good, as the coefficient of variation (CV) was approximately 3 % or less. These results show that it is possible to measure pure water with a low TOC concentration, such as pharmaceutical water, with high accuracy and high sensitivity with both the combustion catalytic oxidation type TOC-L and the wet chemical oxidation type TOC-V.





